Impressive What Is Non Typical Transition Element

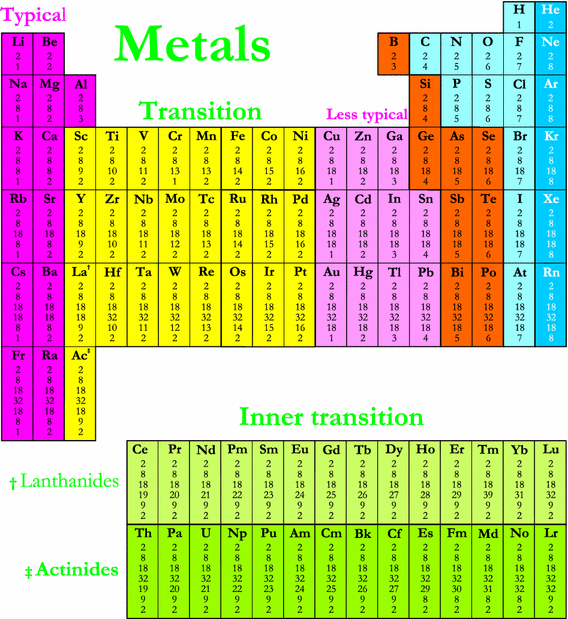
Most transition elements have high melting points and densities so chromium is a typical transition element.
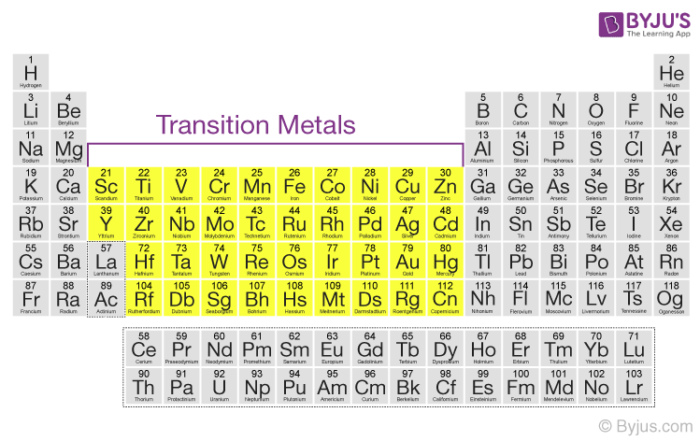
What is non typical transition element. The elements in question are zinc Zn cadmium Cd and mercury Hg. Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table. The group 1 elements have low melting points and densities so sodium is a typical.
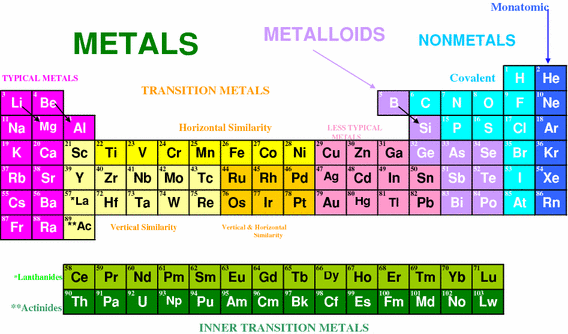
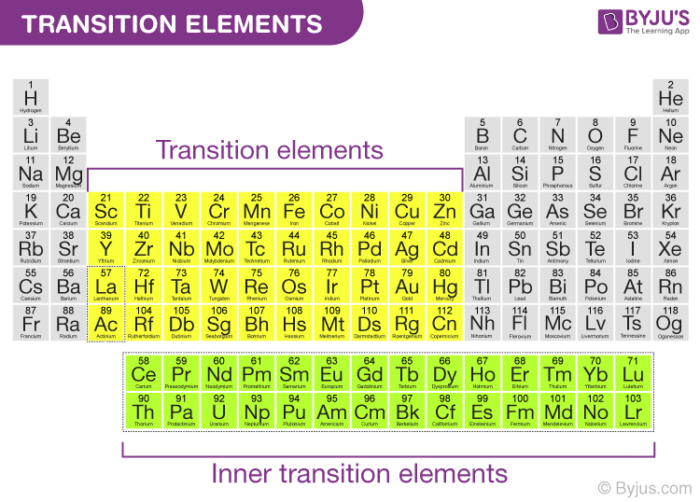
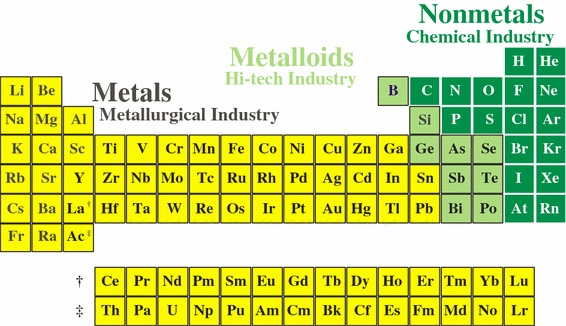
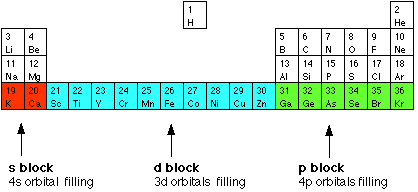
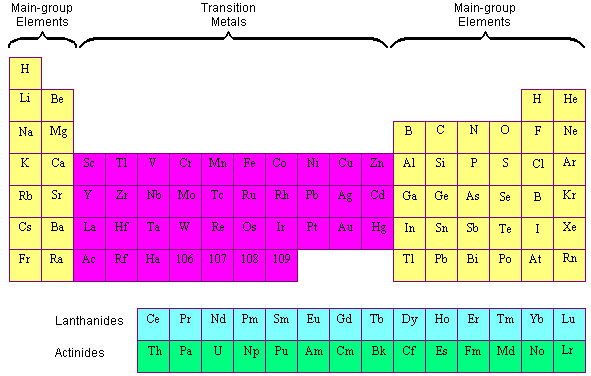
A compound contain metallic and non metallic elements. The disagreement about whether these elements should be classified as main group elements or transition metals suggests that the differences between these categories are not clear. Transition elements are the one which has the partially filled d orbitals example Fe CO Ti etc and non-typical transition elements are the one which has completely filled d orbital example Zn Cd Hg because they have completely filled inner or penultimate d-orbitals.
Hence option D is correct. Non transition elements would include the s-block and the p-block elements including zeroth group noble gases. Anions which contain a non-bonding pair of electrons.
The only oxidative state which zinc has is Zn 2 in which its configuration is Ar 4s 0 3d 10 as the 4s sub-level empties first. What is typical and non-typical transition elements. The definition of a transition metal is that it must have an incomplete d sub-level in one or more of is oxidation states.
Group IIB elements Zn CdHg dont have partially filledd-subshell either in elements or in their ionic ionic state andneither they. These include platinum gold and silver. Non-typical transition elements are d-block elements which have completely filled d-orbitals n-1d10.
Able to form coordinate covalent bonds 6 2 3. The transition metals as a group have high melting points. Some of the more familiar ones are so unreactive that they can be found in nature in their free or uncombined state.